Unveiling the Magnetic Secrets of Spinel Ferrite Nanoparticles
Imagine a world where the secrets of tiny particles could unlock revolutionary advances in magnetic technologies. Henrik Lyder Andersen from the Materials Science Institute of Madrid (ICMM-CSIC) took a step closer to this reality together with researchers at iMAT by uncovering how hydrothermal synthesis allows the atomic arrangement of spinel ferrite nanoparticles to be trapped in a metastable configuration thereby altering their magnetic properties.
The study, recently published in CrystEngComm, utilized advanced X-ray as well as neutron powder diffraction to uncover the distribution of metal cations in the crystal lattice and their impact on magnetic structure and behavior.
Structural Insights into Spinel Ferrites
Spinel ferrites are a fascinating class of materials known for their magnetic versatility. They have a range of current and potential future high-tech applications ranging from data storage to catalysis and biomedical devices. Their unique magnetic properties originate from how metal ions (cations) are distributed across two specific positions in their crystal lattice:
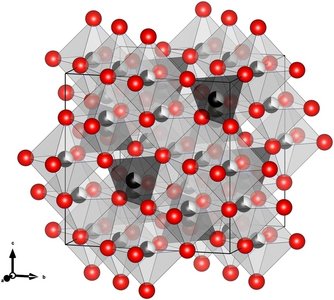
- Tetrahedral (A) sites, surrounded by four oxygen atoms (shown in the figure as dark gray shaded areas)
- Octahedral (B) sites, surrounded by six oxygen atoms (shown in the figure as light gray shaded areas)
The study focused on four spinel ferrite types, i.e. MnFe₂O₄, CoFe₂O₄, NiFe₂O₄, and ZnFe₂O₄, which were synthesized via hydrothermal methods. The researchers analyzed the degree of inversion, which describes how cations are distributed between A and B sites in the spinel structure. They found that the inversion degrees in the nanosized materials differ from the thermodynamically stable configurations found in bulk crystals of the same compounds. This control of the inversion degree unlocks a new handle for tuning the magnetic performance of spinel ferrite nanoparticles.
How Did They Do It?
The researchers combined cutting-edge tools to reveal the atomic and magnetic secrets of previously synthesized nanoparticles:
-
Powder X-ray Diffraction (PXRD) and Neutron Powder Diffraction (NPD): Modelling of both PXRD and NPD data allowed precise determination of the cation distribution between the A and B sites, as well as extraction of the magnetic ordering in the structures.
-
Scanning Transmission Electron Microscopy (STEM-HAADF): Provided atomic-scale images of the nanoparticles, showing their size, shape, and elemental distribution.
-
Vibrating Sample Magnetometry (VSM): Measured the magnetic properties (coercivity, remanence, saturation magnetization) of the nanoparticles by detecting the magnetic moment as a function of an applied magnetic field.
Together, these techniques provided a comprehensive understanding of the structural and magnetic properties of the spinel ferrite nanoparticles.
Key Findings
The researchers synthesized and studied MnFe₂O₄, CoFe₂O₄, NiFe₂O₄, and ZnFe₂O₄ nanoparticles, each displaying distinct structural and magnetic properties. These differences are linked to their composition, cation distribution, crystallite size, and magnetic behavior, as revealed by advanced characterization techniques.
Why does it matter?
Understanding how atomic arrangements influence magnetic properties isn’t just an academic exercise. It is a step toward creating smarter and more efficient materials. This work shows how material synthesis and advanced measurement techniques can provide a deeper understanding of material behavior.
Interested?
You can explore the full study in CrystEngComm to delve deeper into the structural and magnetic properties of spinel ferrite nanoparticles. Otherwise, feel free to explore the other research topics we work on at iMAT.
If you are working on related materials or applications, we invite you to reach out to our Center Manager to discuss potential collaborations or shared research opportunities.
Henrik L. Andersen*, Matilde Saura-Múzquiz, Cecilia Granados-Miralles, Rebekka Klemmt, Espen D. Bøjesen and Mogens Christensen