Surface Chemistry Meets Application: Joint Research Maps Nickel Electrode Evolution for Hydrogen Production
Two PhD students from different departments at Aarhus University joined forces to study how the surface chemistry of industrial nickel foam electrodes change during water splitting. Their combined expertise made it possible to monitor the surface in real time under realistic conditions using advanced spectroscopy techniques.
Producing hydrogen through water splitting is one of the most promising approaches to store renewable energy. Nickel foam is widely used as an electrode material because it is chemically stable, cost-effective, and due to the pores, a high surface area material. However, much remains unknown about what happens on the surface of these electrodes during operation.
In this study, researchers used synchrotron sourced near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) to observe the surface chemistry of nickel electrodes during both hydrogen and oxygen evolution reactions. The work was made possible through collaboration between PhD students Ramadan Chalil Oglou from the Interdisciplinary Nanoscience Center (iNANO) and Morten Linding Frederiksen from the Department of Biological and Chemical Engineering conducting experiments at MaxIV laboratory in Lund, Sweden. By combining surface science and electrochemical engineering, they developed a practical approach to study real electrodes under working conditions.
Investigating Real Electrodes While They Operate
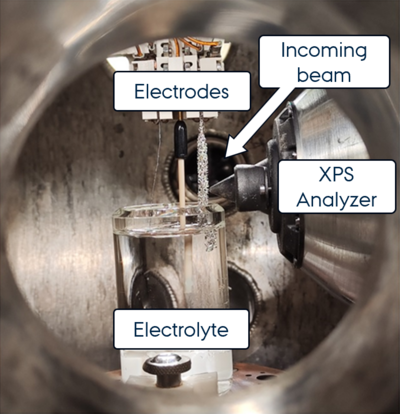
Nickel foam electrodes are not flat or idealized. Their porous, three-dimensional structure makes them excellent for industrial use but difficult to analyze using traditional surface science tools. To solve this, the team used a custom electrochemical cell that allowed them to study the electrode surface with XPS while it was actively running shown in Figure 1.
This setup made it possible to compare what happens during the two main reactions involved in water splitting: the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). Under each condition, the surface of the nickel changed, forming different types of hydroxides and oxides. These surface transformations are closely linked to how well the electrode performs and how long it lasts.
The study showed that under oxygen evolution conditions, the nickel surface became enriched with oxidized species, forming layered hydroxide structures that can influence activity and stability. In contrast, during hydrogen evolution, the surface remained more metallic but still underwent subtle changes that could affect long-term performance. These observations suggest that the chemical environment plays a direct role in reshaping the catalyst surface, and that tailored surface states may be key to improving efficiency in both reactions.
How did they do it?
To understand the chemical changes taking place on the nickel surface, the researchers combined electrochemical control with real-time XPS measurements:
- Near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) measurements allowed them to study the surface during water splitting without removing the sample from the reaction environment.
- A custom electrochemical cell within NAP-XPS chamber enabled simultaneous electrochemical control and XPS analysis of an industrial electrode.
- Surface analysis during HER and OER: They performed in situ comparisons under both hydrogen and oxygen evolution reactions to see how the surface responded to different polarizations.
The approach offers a new way to study electrode materials in action rather than only before or after operation.
Key Findings
Their work showed a successful example of how combining methods across departments can lead to new research possibilities and furthermore:
- A working method for real-time surface analysis of porous nickel foam electrodes
- Dynamic formation of nickel hydroxides and oxides during water splitting
- Clear differences in surface behavior between hydrogen and oxygen evolution reactions
Why Does It Matter?
Understanding how electrode materials behave during water splitting is essential for improving the production of clean hydrogen. Until now, researchers have often relied on static or post-reaction measurements that do not capture the full picture.
This study demonstrates that it is possible to follow surface changes as they happen. The new approach provides valuable insight into how catalysts function and how they degrade. This can help researchers and industry design better materials for long-term use in electrolysis systems.
Interested?
You can explore the full study in The Journal of Physical Chemistry Letters. Otherwise, feel free to explore the other research topics we work on at iMAT.
If you work in electrochemistry, surface science, or hydrogen technologies, we welcome collaborations and further discussion. 📨 Reach out to our Center Manager.
Ramadan Chalil Oglou, Morten Linding Frederiksen, Zhaozong Sun, Marcel Ceccato, Andrey Shavorskiy, Jeppe Vang Lauritsen
