How Changing the pH Controls the Growth and Performance of BiOX Nanomaterials
New research investigates how pH-controlled synthesis alters the shape, stacking, and lattice distortion in BiOX (X = Cl, Br, I) nanomaterials. The study provides key insights into how morphology and microstrain can be tuned to optimize catalytic and photocatalytic applications.
Bismuth oxyhalides (BiOX) are layered nanomaterials with promising applications in catalysis and photocatalysis. Their physical and chemical behavior is linked to their crystal structure and how individual atoms are arranged. In a recent study published in ACS Applied Nano Materials, researchers investigated how varying the synthesis pH affects the microstructure, crystallite thickness, and lattice distortion in BiOCl, BiOBr, and BiOI. The research team, consisting of 3 different iMAT groups, used a microwave-assisted synthesis method to obtain ultrathin nanocrystals followed by a suite of advanced characterization techniques to morphology and atomic-level structural configurations evolve with pH.
Controlling Lattice Structure Through Synthesis
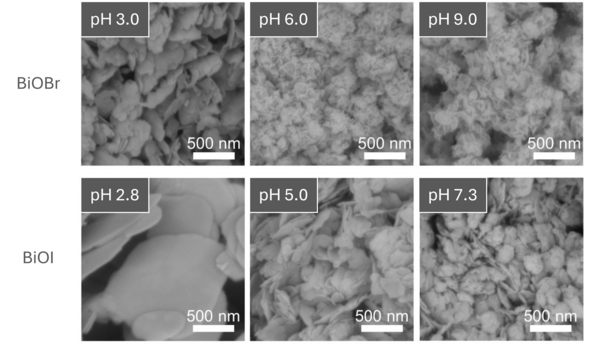
Regardless of the halide used, the BiOX samples formed platelet-like crystallites dominated by the exposure of {001} facets as shown in the scanning electron images in Figure 1. However, their size, stacking, and internal strain were found to vary significantly with the pH of the precursor solution:
- At higher pH, the crystallites formed were thinner, with stacking-direction thicknesses as low as 3-5 nm.
- These ultrathin materials showed signs of increased microstrain and lattice distortion, due to reduced dimensional stability.
- In contrast, samples synthesized at lower pH showed greater crystallite thickness and lower strain.
How Did They Do It?
To map out the structure of these materials in detail, the team used multiple complementary techniques:
- Rietveld refinement of powder X-ray diffraction (PXRD) and X-ray pair distribution function (PDF) analysis provided precise information about unit cell dimensions, crystallite size, and microstrain.
- X-ray absorption spectroscopy (XAS) was used to confirm that the local bonding environments did not change significantly across the different pH conditions.
- Diffuse reflectance spectroscopy (DRS) and DFT calculations demonstrated that the band gap remained mostly unchanged, indicating that the structural modifications affect lattice strain without directly impacting the electronic structure.
- Surface-sensitive methods like X-ray photoelectron spectroscopy (XPS) and energy-dispersive X-ray spectroscopy (EDX) revealed that the apparent halide deficiency in high-pH samples was likely due to surface effects, not a change in bulk composition.
Key Findings
-
The study revealed several important trends relevant to BiOX design:
-
Synthesis pH controls crystallite thickness and microstrain, providing a pathway to tailor the morphology of BiOX nanomaterials without altering their bulk phase.
-
Ultrathin BiOX materials produced at higher pH are more strained and structurally distorted but retain their crystal orientation favorable for photocatalysis.
-
The materials remain chemically stable with minimal changes to local bonding or band gap properties across different synthesis conditions.
Why Does It Matter?
Understanding how synthesis conditions shape the physical and structural properties of BiOX nanomaterials allows researchers to design materials with improved surface area and catalytic behavior. The ability to tune crystallite thickness and strain without compromising chemical stability is crucial for optimizing BiOX materials in applications such as photocatalysis, environmental remediation, and solar energy conversion.
The work highlights the importance of combining synthetic control with advanced structural analysis to uncover the hidden relationships between structure and performance in layered materials.
Interested?
You can explore the full study in ACS Applied Nano Materials. Otherwise, feel free to explore the other research topics we work on at iMAT.
If you are working on related materials or applications, we invite you to reach out to our Center Manager to discuss potential collaborations or shared research opportunities.
Melissa Jane Marks, Cecilie Friberg Klysner, Sara Frank, Nanna Nielsen Lange, Rebekka Klemmt, Henrik Særkjær Jeppesen, Marcel Ceccato, Espen Drath Bøjesen, Maarten G. Goesten, Nina Lock