Transforming Coordination Polymers and Metal-Organic Frameworks into Functional Catalysts
PhD student Sara Frank from Aarhus University has investigated how metal-organic framework materials change during electrochemical reactions and how these transformations can be used to design better catalysts. Her work combines synthesis, structural analysis, and real-time measurements to explore how functional catalysts form and perform. The findings are relevant for developing materials used in sustainable technologies such as carbon dioxide conversion and water splitting.
Electrocatalysis plays a key role in converting electricity into chemical fuels. These reactions are part of energy technologies that aim to reduce carbon emissions, such as the electrochemical reduction of carbon dioxide and the oxygen evolution reaction. However, many electrocatalysts are not stable under working conditions. They transform into other materials during operation, which makes them difficult to study and optimize. Sara’s PhD project addresses this challenge by tracking materials as they change and understanding how these changes relate to performance.
From Metal-Organic Precursors to Scalable Catalyst Solutions
Sara investigated how to turn metal-organic compounds into effective catalysts using heat and electrochemical activation. She focused on low-cost and tunable materials known as coordination polymers (CPs) and metal-organic frameworks (MOFs). MOFs are a special type of coordination polymer made from metal atoms connected by organic molecules, forming a highly porous 3D network. These materials are especially promising for use in electrocatalysis because their pores provide space for reactions, their metal atoms are well separated, and their structure can be designed in advance. A key advantage is that they can also be transformed into active catalysts using either electricity or controlled heating.
Her results showed that even materials that are chemically unstable at first can become highly active during use. Instead of aiming for stability from the start, the key is to select precursors that transform in the right way under operating conditions.
One approach in her work involved heating the coordination polymer-based materials under well-defined conditions. The transformation of a MOF into an active catalyst is illustrated in the sequence at the bottom of Figure 1. This thermal treatment made it possible to control the nanostructure and catalytic activity of the resulting materials, allowing them to perform better in electrochemical reactions such as carbon dioxide reduction.
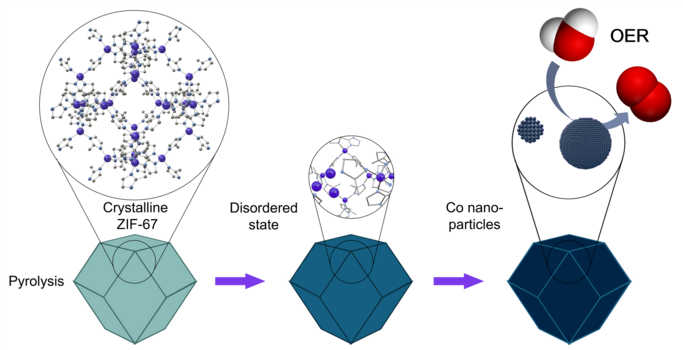
For industry, this research points toward a possible pathway for producing tailored catalyst materials from simple precursors through scalable thermal processes. While further development is needed, the results suggest that with precise control, it may become feasible to reduce reliance on expensive or highly stable starting materials, opening more flexible and cost-effective routes in the future.
AUREX: Watching Catalysts in Real Time
To understand what happens during catalysis, Sara developed, together with colleagues, a new electrochemical cell for real-time observations of experiments. The Aarhus University Reactor for Electrochemical studies using X-rays (AUREX) cell allows researchers to observe changes in material structure during electrochemical reactions using synchrotron-based X-ray techniques as can be seen in Figure 2.
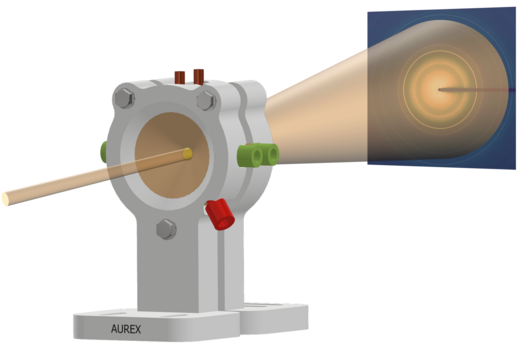
Sara used this setup to monitor several types of catalyst systems. These included both commercial materials and coordination polymer samples she prepared herself. The experiments showed that both the initial structure and the surrounding chemical environment affect how the catalyst evolves and how well it performs. This approach provides insight into how active structures form and which transformations are beneficial for catalytic activity.
How Did They Do It?
Sara’s work combined electrochemical and advanced structural characterization techniques to study catalysts at different stages of their transformation. She used:
- Thermal conversion (pyrolysis): Heating coordination polymers and MOFs to create nanostructured catalysts.
- X-ray synchrotron techniques: Sara used different X-ray methods to study how the catalysts changed during use. X-ray absorption showed how the chemical environment around specific elements changed. Total scattering and X-ray diffraction revealed changes in atomic structure at both short and long distances, helping to track how the material transformed during operation.
- Electrochemical testing: Measuring how well different coordination polymers performed during reactions by testing them under conditions similar to those used in real devices.
Key Findings
- Unstable coordination polymers can lead to high-performance electrocatalysts after transformation in reaction conditions
- Another route to achieve active catalysts out of coordination polymers is controlled heating to obtain new nanomaterials with improved activity and stability
- Real-time measurements revealed how catalyst formation depends on both precursor identity and reaction environment. Operando measurements are essential for understanding active structures under realistic conditions
Why Does It Matter?
Many promising electrocatalysts do not retain their original structure during operation. Sara Frank’s work shows that these transformations are not only common but can be beneficial. By understanding how catalysts form and evolve, it becomes possible to design better materials that work in real systems.
Her results support the development of efficient and stable catalysts for carbon dioxide conversion and water splitting. They also highlight the importance of real-time measurements in materials science and demonstrate how flexible synthesis pathways can lead to effective solutions for energy technologies.
Lear More and Stay Tuned!
You can find more information about Sara's work in the following publications:
Sara Frank, Erik Svensson Grape, Espen Drath Bøjesen, Rasmus Larsen, Paolo Lamagni, Jacopo Catalano, A. Ken Inge and Nina Lock
Sara Frank, Mads Folkjær, Mads L. N. Nielsen, Melissa J. Marks, Henrik S. Jeppesen, Marcel Ceccato, Simon J. L. Billinge, Jacopo Catalano and Nina Lock
Sara Frank, Marcel Ceccato, Henrik S. Jeppesen, Melissa J. Marks, Mads L. N. Nielsen, Mads Folkjær, Ronghui Lu, Jens Jakob Gammelgaard, Jonathan Quinson, Ruchi Sharma, Julie S. Jensen, Sara Hjelme, Cecilie Friberg Klysner, Simon J. L. Billinge, Justus Just, Fredrik H. Gjørup, Jacopo Catalano and Nina Lock
More publications are in work so stay tuned to learn more!
If you are working with metal-organic materials, carbon-based catalysts, or real-time structural methods, you are welcome to contact the iMAT Center Manager to explore potential collaborations or how these findings can help you.
