Carbonation for Sustainable Cement: How Calcium Silicates and Calcium Aluminates React with CO₂
PhD student Rune Wittendorff Mønster Jensen who’s work at Aarhus University in a research group of iMAT has studied how cement-related materials react with carbon dioxide. His research brings new insight into how CO₂ can be used not just as a problem to solve in the form of emissions, but as a tool during production to strengthen future building materials.
Cement is essential for building homes, bridges, and cities. But producing it is one of the biggest industrial sources of carbon dioxide (CO2) emissions. To make cement more climate-friendly, researchers are exploring new types of materials that can react with CO2 instead if just of releasing it. One promising idea is to let the cement absorb CO2 during the hardening process, which is called carbonation. This is different from the traditional way cement hardens, which relies on water and is known as hydration.
In hydration, cement reacts with water to form a substance that holds the concrete together. In carbonation, cement-like materials react with CO2, forming solid minerals like calcium carbonate (CaCO3). This method has the potential to trap CO2 while also producing strong building materials.
Learning What Happens During Cement Carbonation
In his PhD project, Rune studied how different building material components react with CO2. He looked closely at two important groups: calcium silicates and calcium aluminates. Calcium silicates are the main ingredients in traditional cement, while calcium aluminates (Ca3Al2O6) are used to help cement set quickly.
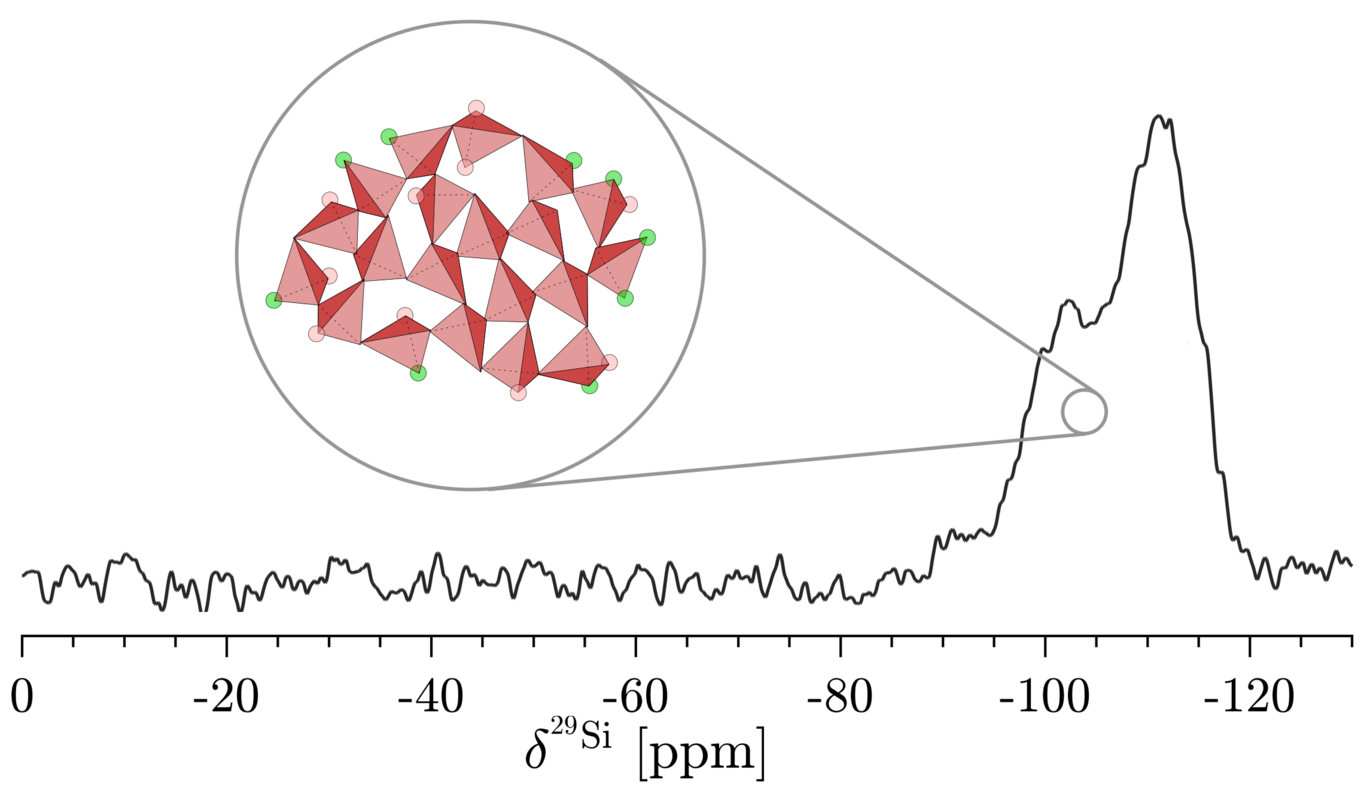
To observe what happens during carbonation, Rune used a method called wet, enforced carbonation. This means the materials were exposed to CO2 in the presence of water under controlled lab conditions. The resulting reaction products can be seen in Figure 1 for the different starting materials. He used advanced tools such as Nuclear Magnetic Resonance (NMR) spectroscopy, thermogravimetric analysis (TGA), and infrared (IR) spectroscopy to study how the materials changed over time.
How Did They Do It?
To understand how different cementitious materials carbonate, the research applied several specialized techniques:
- NMR spectroscopy: Identified the structure of silica gel and various types of CaCO3 over time.
- TGA measurements: Provided insight into the amount of CaCO3 being formed
- IR spectroscopy: Complemented the NMR analysis by verifying the type of CaCO3 present
Through this approach, it was possible to track how carbonation product developed from early hydrated phases to the fully carbonated products and how different materials favored specific pathways depending on their structure and solubility.
He found that most calcium silicates reacted well with CO2. In just a few hours, they turned into solid products like calcite, a mineral form of calcium carbonate, and a type of silica gel. Some materials, like CaSiO3, needed more time and a significantly higher temperature of 60 °C (instead of room temperature) to carbonate.

One key observation was that some materials started out by reacting with water (hydration) before reacting with CO2. This was especially true for triclinic Ca3SiO5, which showed a burst of hydration in the first 20 minutes, helping kick-start the carbonation process. Other materials, like Ca3Si2O7, more prone to directly reacting with CO2.
Key Findings
- Most importantly, the lower calcium containing calcium silicates, except CaSiO3, gave rise to the exact same carbonated product, a highly polymerized silica gel and calcite, with very comparable reaction time.
- Most calcium silicates quickly formed reaction products in the presence of the CO2 saturated solution, showing good potential for use in CO2-hardened cements.
- Some materials, such as triclinic Ca3SiO5, began reacting with water first, while others, such as Ca3Si2O7 reacted directly with CO2.
- Furthermore, surprising findings for the carbonation of calcium aluminates were discovered. The results will be published at a later point and will not be described here.
Why Does It Matter?
This research helps explain how new types of cement materials can be designed to absorb CO2 during production instead of merely releasing it. By understanding which materials react best, and how they do so, we can begin creating concrete and cement products that are both strong and climate friendly.
The study also shows that hydration and carbonation can work together, and that different materials follow different pathways during hardening. Even though, the resulting products can still be very similar. This is important when choosing ingredients for future applications of cement.
What’s Next? Stay Tuned!
Further results of Runes PhD are currently in preparation for publication. Stay tuned for more as the findings are released later this year!
If you are working on related materials or applications, we invite you to reach out to our Center Manager to discuss potential collaborations or shared research opportunities.
